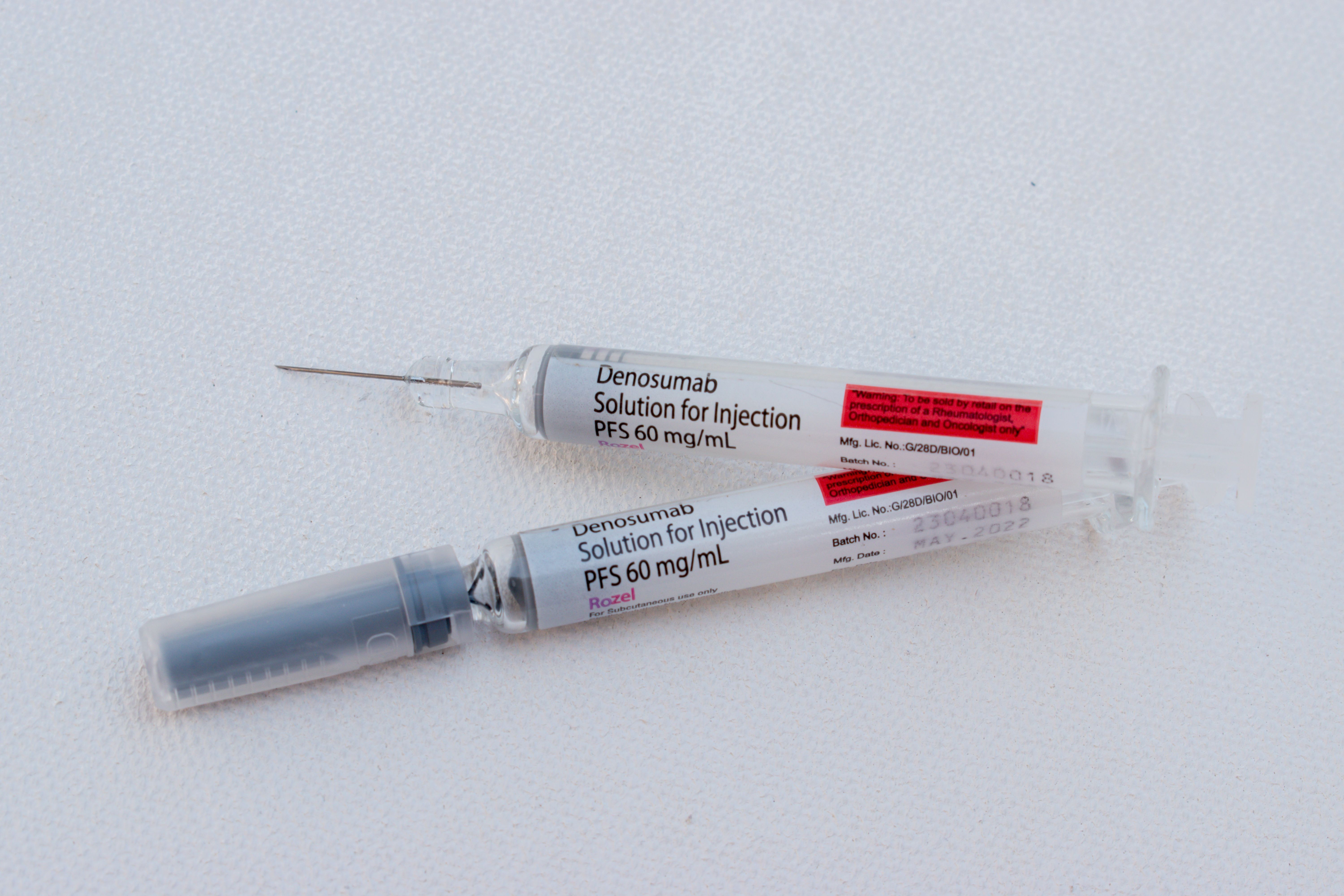
Two Biocon Biologics Denosumab Biosimilars Approved by MHRA
Biocon Biologics, headquartered in India, has announced that two biosimilars it has developed referencing denosumab have been granted marketing authorization in the United Kingdom by the Medicines and Healthcare products Regulatory Agency (MHRA) (1). The …