
Artificial Blood Vessels Market Projected to Hit USD 10.0 Billion by 2035, at a Exceptional CAGR 5.89%
Artificial Blood Vessels Market
Smart Polymers and Bioactive Coatings: These materials help reduce thrombosis, promote healing, and improve long-term patency rates.
US, NY, UNITED STATES, April 15, 2025 /EINPresswire.com/ -- Artificial Blood Vessels Market: Engineering the Future of Cardiovascular Treatment
Introduction
Cardiovascular diseases remain the leading cause of mortality worldwide, with millions of individuals requiring surgical intervention each year. Among the most critical innovations in vascular surgery is the development of artificial blood vessels, which offer a life-saving alternative to traditional vein grafts. These synthetic or bioengineered conduits are used to restore blood flow in patients suffering from blocked or damaged arteries and veins, especially when suitable natural grafts are unavailable. As healthcare technology advances, the artificial blood vessels market is poised to become a vital segment of cardiovascular care.
With rising cases of peripheral artery disease, coronary artery disease, and vascular trauma, the demand for reliable and durable vascular grafts has significantly increased. The global market for artificial blood vessels is expected to grow steadily, driven by aging populations, rising chronic disease incidence, and advancements in tissue engineering.
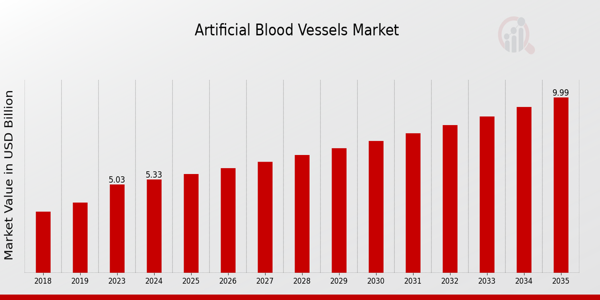
Artificial Blood Vessels Market Overview
As per MRFR analysis, the Artificial Blood Vessels Market Size was estimated at 5.03 (USD Billion) in 2023. The Artificial Blood Vessels Market is expected to grow from 5.33 (USD Billion) in 2024 to 10.0 (USD Billion) by 2035. The Artificial Blood Vessels Market CAGR (growth rate) is expected to be around 5.89% during the forecast period (2025 - 2035).
🔍 Download Sample Report
https://www.marketresearchfuture.com/reports/artificial-blood-vessels-market-42960
Market Dynamics
The artificial blood vessels market is being propelled by a mix of clinical needs, technological progress, and patient-specific applications. Traditionally, surgeons relied on autologous veins, such as the saphenous vein, for bypass procedures. However, many patients do not have suitable veins for grafting due to age, prior surgeries, or comorbidities. Artificial vessels are stepping in to bridge this gap.
Advanced materials such as expanded polytetrafluoroethylene (ePTFE), Dacron, and polyurethane have enabled the creation of vessels that mimic the biomechanical properties of natural arteries. These materials resist blood clotting, support endothelial cell growth, and reduce the risk of infection. Moreover, tissue-engineered blood vessels, which are created using stem cells and scaffolds, represent the frontier of regenerative medicine in vascular surgery.
Clinical Applications and Use Cases
Artificial blood vessels have been successfully used in a range of surgical procedures. The most common applications include:
Coronary artery bypass grafting (CABG) for patients with coronary artery disease.
Hemodialysis access for patients with end-stage renal disease.
Peripheral artery bypass procedures for those with limb ischemia.
Congenital heart defect repairs in pediatric patients.
Trauma or injury-related vascular reconstructions.
With the growing popularity of minimally invasive techniques, artificial vessels are now being adapted for compatibility with endovascular stenting and catheter-based procedures. This enhances patient outcomes by reducing recovery time and surgical complications.
Technological Advancements Shaping the Market
Recent innovations are making artificial blood vessels safer, more durable, and biologically compatible. Some of the key advancements include:
Tissue-Engineered Vessels: Researchers are developing vessels using biodegradable scaffolds seeded with the patient’s own cells. These vessels integrate better with host tissues and have lower rejection risks.
3D Bioprinting: This cutting-edge technique allows for the fabrication of custom-shaped blood vessels tailored to the patient’s anatomy.
Smart Polymers and Bioactive Coatings: These materials help reduce thrombosis, promote healing, and improve long-term patency rates.
Drug-Eluting Grafts: These are coated with anti-inflammatory or antiproliferative drugs to reduce restenosis and improve healing.
Such advancements are significantly expanding the market potential and clinical appeal of artificial blood vessels.
Market Drivers
Several global factors are accelerating the demand for artificial blood vessels:
Rising Cardiovascular Disease Prevalence: As lifestyles become more sedentary and populations age, the burden of heart and vascular diseases continues to rise.
Shortage of Suitable Donor Vessels: Many patients do not have healthy veins or arteries suitable for autografts, making synthetic alternatives essential.
Growing Number of Surgeries: The increasing volume of vascular and cardiac surgeries, including CABG and dialysis access procedures, fuels demand.
Technological Innovation: Improved materials and biocompatibility features are making artificial vessels more reliable and effective.
Emergence of Regenerative Medicine: The integration of tissue engineering and stem cell research is enhancing the success rate of bioengineered vessels.
Market Challenges
Despite the promising growth, the artificial blood vessels market also faces notable challenges:
Risk of Infection and Thrombosis: Artificial materials can trigger clot formation or infections if not properly managed.
Limited Patency in Small Diameter Grafts: Artificial vessels perform well in large-diameter applications but tend to fail in smaller vessels due to thrombosis.
High Cost of Development: Bioengineered vessels and custom-designed grafts involve significant research and production costs.
Regulatory Hurdles: Stringent approval processes for novel graft materials and bioengineered products can delay market entry.
Overcoming these challenges will be crucial for wider adoption and market expansion.
🛒 You Can Purchase Complete Report
https://www.marketresearchfuture.com/checkout?currency=one_user-USD&report_id=42960
Regional Insights
North America leads the artificial blood vessels market, owing to advanced healthcare infrastructure, a high prevalence of cardiovascular diseases, and rapid adoption of cutting-edge medical technologies. The U.S., in particular, has seen strong investment in regenerative medicine and vascular surgery solutions.
Europe follows closely, with countries like Germany, France, and the UK focusing on innovation and collaborative research initiatives. Favorable reimbursement policies and government support for medical research also boost the regional market.
Asia-Pacific is emerging as a lucrative growth region, with rising healthcare expenditure, increasing patient awareness, and large untapped markets in countries like China, India, and Japan. Rapid urbanization and lifestyle changes are also contributing to higher cardiovascular disease rates, thereby increasing demand for vascular interventions.
Key Companies in the Artificial Blood Vessels Market
Cook Medical
Abbott Laboratories
Johnson and Johnson
B. Braun Melsungen
Integra LifeSciences
St. Jude Medical
Smith and Nephew
CryoLife
Boston Scientific
Sorin Group
Tissue Regenix
Cardinal Health
Stryker
Terumo
Medtronic
These companies are at the forefront of developing innovative vascular grafts, combining engineering precision with biological compatibility.
Artificial Blood Vessels Market Segmentation Insights
Artificial Blood Vessels Market Product Type Outlook
Synthetic Blood Vessels
Bioengineered Blood Vessels
Decellularized Blood Vessels
Artificial Blood Vessels Market Material Outlook
Polymers
Composites
Biomaterials
Artificial Blood Vessels Market Application Outlook
Cardiovascular Surgery
Transplant Surgery
Trauma Surgery
Artificial Blood Vessels Market End User Outlook
Hospitals
Ambulatory Surgical Centers
Research Laboratories
Artificial Blood Vessels Market Regional Outlook
North America
Europe
South America
Asia Pacific
Middle East and Africa
Key Inquiries Addressed in This Report:
What are the most common clinical applications of artificial blood vessels?
How are tissue-engineered and 3D-printed blood vessels changing the market?
What factors are driving the demand for artificial blood vessels globally?
Which challenges need to be addressed to improve long-term graft success?
How do regional dynamics influence market growth?
Who are the leading players in this space, and what innovations are they introducing?
Related MRFR Reports with Full Detailed Analysis:
South Korea Peripheral Nerve Stimulators Market: https://www.marketresearchfuture.com/reports/south-korea-peripheral-nerve-stimulators-market-44904
Spain Peripheral Nerve Stimulators Market: https://www.marketresearchfuture.com/reports/spain-peripheral-nerve-stimulators-market-44913
Uk Peripheral Nerve Stimulators Market: https://www.marketresearchfuture.com/reports/uk-peripheral-nerve-stimulators-market-44903
Us Peripheral Nerve Stimulators Market: https://www.marketresearchfuture.com/reports/us-peripheral-nerve-stimulators-market-12836
Tranexamic Acid Market: https://www.marketresearchfuture.com/reports/tranexamic-acid-market-33541
China Robot Assisted Surgical Systems Market: https://www.marketresearchfuture.com/reports/china-robot-assisted-surgical-systems-market-45590
France Robot Assisted Surgical Systems Market: https://www.marketresearchfuture.com/reports/france-robot-assisted-surgical-systems-market-45585
Gcc Robot Assisted Surgical Systems Market: https://www.marketresearchfuture.com/reports/gcc-robot-assisted-surgical-systems-market-45586
Germany Robot Assisted Surgical Systems Market: https://www.marketresearchfuture.com/reports/germany-robot-assisted-surgical-systems-market-45583
India Robot Assisted Surgical Systems Market: https://www.marketresearchfuture.com/reports/india-robot-assisted-surgical-systems-market-45589
Market Research Future
Market Research Future
+1 855-661-4441
email us here
Visit us on social media:
Facebook
X
LinkedIn
YouTube
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release